The Best Bang for Your Buck Supplements
by Alden Ryno on May 15, 2015The Best Bang for Your Buck Supplements
In the growing pursuit of balancing performance and health, we physique and strength athletes are inundated with a pantry full of vitamins, minerals, and other supplements in the form of pills, powders, and capsules. Instead of searching for a specific compound for a specific ailment or benefit, we should be looking into the compounds that give us the most bang for the buck! For example, Vitamin D is touted for positive effects on immune function, bone health, insulin sensitivity, systemic inflammation, and brain chemistry balance…just to name a few. Are there potentially other supplements that aren’t vitamins or essential minerals, but we’ll greatly benefit from? Absolutely! This one, in particular, is probably in your cabinet already. If it’s not, then you’ve almost certainly heard of it.
Before disclosing this mystery supp, let’s explore several of its known roles in the human body: Essential to glucose metabolism1,2; initiation and enhancement of GLUT4 translocation3–5; expression of insulin receptor substrate 1 (IRS1) and insulin sensitization3,6; drive nutrients to skeletal muscle and away from adipose tissue6–8; regarded as the most powerful antioxidant and antioxidant regenerator9–12; suppression of chronic inflammation3,13; heavy metal chelation2,14,15; hypotensive agent16–18; and modification of vascular rigidity19,20.
Boy, what a list! Seriously, look back at it. Do you have any idea of what compound I’m talking about? Alpha-lipoic acid. Probably not what you thought, but why? Why aren’t we told to supplement with ALA? Because we produce it naturally….just enough to get by.
As physique and performance athletes, we shouldn’t just want to “get by” though. We should strive to be in an optimal state of being through effective nutrition, exercise, and supplementation. And I’m going to show you how and why (if you’re not already convinced) you should supplement with our new best friend; α-lipoic acid.
What makes it special?
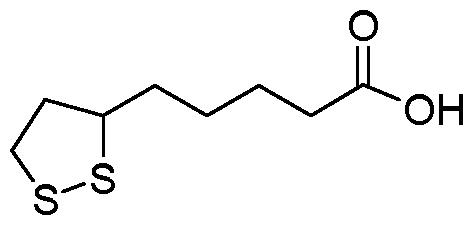
First of all, the structure of ALA is relatively simple, however, quite versatile. Chemically speaking, ALA is interesting because it is both water and fat soluble. This means that is can be absorbed, metabolized, and stored in numerous ways whereas most compounds are either water soluble OR fat soluble.
Figure 1: The chemical structure of ALA. Analogous to a medium chain fatty acid.
Vitamins A, D, E, and K are fat soluble; they are stored in liver and fatty tissue in the body. We can store the vitamins for extended periods of time and, as a result, can build up an excess of them, increasing the ability of toxicity.
Water soluble vitamins, such as vitamins C and B, are not stored in body tissues and rapidly pass through the body. We need a relatively constant supply of these vitamins so we don’t suffer from deficiencies. Explorers crossing the Atlantic Ocean, for example, developed scurvy after a couple of months due to a lack of vitamin C from insufficient consumption of fresh fruit. However, a benefit of these water soluble vitamins is that the potential for toxic doses is extremely low.
According to Ziegler et al. doses of 1800 mg/day of ALA administered for 6 months showed no adverse effects21. This is great because the effective dose of ALA is much lower than this. While there are no established levels of toxicity for ALA, it has been shown that as much as 98% of ingested ALA is excreted by the body within 24 hours22….meaning daily supplementation is required to reap the continued benefits of ALA.
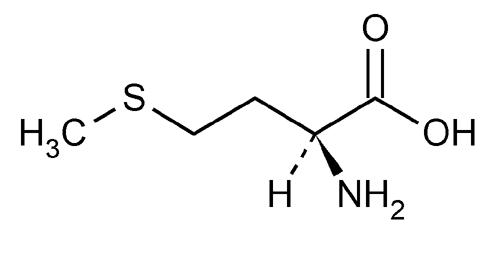
Alpha-lipoic acid is also distinctive because of the sulfur atoms in its structure. Sulfur is a relatively large and “squishy” (actual technical term) atom. This allows sulfur to create be easily oxidized and, subsequently, reduced and to create special, disulfide bonds and bridges with and inside of proteins, specifically those with the amino acids methionine and cysteine (shown below). Disulfide bonds are commonly seen in proteins in order to help shape the proteins as well as allow the proteins (as enzymes) to bind to particular substrates.
 |
 |
Figure 2: Methionine and Cysteine
Another interesting chemical aspect of ALA is that both its oxidized (ALA) and reduced (DHLA) forms are active in the body and are useful in exhibiting effects on the body14! This contrasts many other supplements such as vitamin D in which only a single metabolite is active in the body. We’ll discuss some specific benefits of this unique quality later. For now, just know that this is good since our body can use ALA for one function, then use one of its metabolites (such as DHLA) for another process; a little goes a long way.
Figure 3: Dihydrolipoic Acid (DHLA), the reduced form of ALA.
Whattit do?
Now, we can get into the multitude of benefits from ALA supplementation!
We all know that we don’t grow in the gym. In fact, we break ourselves down. While some degree of breakdown and oxidative stress is essential if we can’t get that breakdown under control, then we can suffer from excessive oxidative stress. This occurs when the production of reactive oxygen species (ROS), reactive nitrogen species (RNS) and other radical species overwhelm the body’s ability to produce antioxidants. While we do need ROS in order for proper cell function, there is always a limit when things get bad. This is why we always hear about antioxidants; they prevent excessive cell damage due to rogue ROS. Studies have shown that oxidation-reduction (redox) couple between ALA:DHLA is one of the most powerful antioxidant combinations9,11,23. However, the results haven’t been shown to directly correlate in vivo (in living tissue). Fortunately for us, ALA also displays an indirect antioxidation method by rapidly regenerating other endogenous antioxidants such as vitamins C and E, but it appears to be best at regenerating glutathione24–26. To quote Han et al. “lipoic acid enables the key enzyme of glutathione synthesis, gamma-glutamylcysteine synthetase, which is regulated by uptake-limited cysteine supply, to work at optimum conditions.” ALA has also shown to increase glutathione concentrations in many cells ranging throughout the body; from T cells (a type of white blood cell) to glial cells (which protect neurons and the CNS)26. For those of you that supplement glutathione for liver health and antioxidant effects, supplemental ALA may be a way to drastically save money.
In concert with ALA’s ability to regenerate endogenous antioxidants, it is also able to chelate numerous heavy metals, including lead, mercury, cadmium, and arsenic2,14, which are extracted from the body via binding to glutathione, ALA, and DHLA. But why should a health athlete such as yourself worry about heavy metals? Other than for obvious health concerns, it’s because these metals are often stored in the liver, the kidneys, and adipose tissue. When attempting to lose bodyfat, these heavy metals (and other stored toxins) are released during lipid peroxidation/fatty acid oxidation, partially as a result of ROS (curbed by ALA). We need for our filters (liver and kidneys) to be functioning properly for optimal health and fat loss.
Like many areas of research, ALA’s efficacy in riding the body of these heavy metals is mixed; some studies show very promising results while others don’t demonstrate a statistical significance. I believe that ALA should definitely be considered for its potential benefits of heavy metal chelation since two major chelation techniques, DMPS and DMSA, can become quite toxic when administered incorrectly while ALA does have several studies supporting its role in removing mercury from the liver, kidneys, and brain at higher doses; which as stated earlier, has not shown to have detrimental effects on health27,28.
A second benefit that stems from ALAs ability to regenerate antioxidants, specifically glutathione, is the alleviation of acute and chronic hypertension29. Clinically, ALA has been used in conjunction with acetyl-L-carnitine (ALCAR) to decrease systolic pressure in hypertensive patients while the sole supplementation of ALA did not show a benefit on blood pressure17,18. This has been attributed to the enhanced synthesis of endothelial nitric oxide (NO) and the inhibition of endothelin-1, a vasoconstrictor produced by endothelial tissue. These effects subdue changes vascular in the kidneys. In tandem with its hypotensive effects, the ALCAR/ALA combination has also shown to significantly reduce vascular rigidity, potentially mitigating the build-up of arterial plaque20. Federici et al. postulate that this is achieved through the stimulation of eNOS (endothelial nitric oxide synthase) to generate NO; a regulator of blood vessel wall elasticity19,30. It should also be noted that clinical doses over two weeks of 100mg/kg (8,100 mg for a 200lb man) did show hypotensive effects while 10mg/kg (810 mg for a 200 lb man) did not; thus, the minimum effective dose les between these two limits.
In a similar fashion, to its regenerative metal chelating effects, ALA has strong support to demonstrate that it inhibits the production of LPS-induced (lipopolysaccharide, a.k.a endotoxins) NF-κβ transcription factors31. What? I know, that’s a mouthful. Essentially NF-κβ is associated with numerous chronic inflammatory diseases; rheumatoid arthritis, atherosclerosis, COPD, asthma, MS, IBD, and ulcerative colitis32. While ALA has been shown to inhibit the expression of numerous inflammation related genes, they are almost always traced back to NF-κβ33,34. Unfortunately, a vast majority of these studies did not disclose the dosages administered to subjects. I assume that this is because they were not done in human subjects (dogs, mice, and cats), but none of the doses approached the LD-50 of the test subjects. So it is safe to say that moderate dosages (200-600 mg/day) wouldn’t harm us, especially since these effects have been shown to some effect with only our natural production of ALA.
Glucose, Insulin, and GLUT4, oh my!
These effects are of the most interest to us! Before we even get to insulin and GLUT4, we need to know that we would die without ALA. Through the process of glycolysis our body takes 10 enzymatically driven steps to convert glucose into pyruvate which is then used in the Kreb’s cycle to produce large amounts ATP. ALA acts as a cofactor in the enzymes that convert pyruvate (from glycolysis) into acetyl-coenzyme A to be used in the Kreb’s Cycle3,35. This is why we must naturally produce small amounts of ALA…without it we would not have sufficient amounts of energy to survive. As a quick side note, ALA is synthesized de novo from octatonic acid, a medium chain fatty acid found in coconut oil and breast milk. You can decide how to get it in your diet…
AMPK & PTP1B: Nutrient Partitioning
What’s known is about supplemental ALA is that it has an effect on AMPK (adenosine monophosphate [AMP]-activated protein kinase) and PTP1B (protein tyrosine phosphatase 1B8,36. As we use energy through the consumption of ATP, AMP concentration increases (triggering AMPK). This is significant because AMPK signals insulin to shuttle nutrients to our cells causing the biogenesis of GLUT4 to suck those nutrients into our cells6,37–39. These effects have been noticed for up to 6 hours post-ingestion of 200-600 mg of ALA7,40. The diminishment of PTP1B by ALA is advantageous because of the relation that PTP1B has with insulin. PTP1B acts as a director of where nutrients should go. When there are greater amounts of PTP1B, insulin shuttles more nutrients and triglycerides to adipose tissue, instead of skeletal muscle. Unlike the whole body properties of ALA on PTP1B, the outcome of ALA on AMPK depends on where we look in the body! In skeletal muscle, ALA enhances the use of AMPK, resulting in increased insulin sensitivity, improved GLUT4 activity, and fatty acid oxidation1,39. If we go to the hypothalamus, then the effects are reversed… Here ALA suppresses AMPK activity leading to decreased brain energy expenditure and suppressing appetite, thus exhibiting anti-obesity effects41! I would be remiss if I didn’t say that the effects of ALA on insulin and GLUT4 are delayed compared to just insulin and are primarily exhibited by the R-stereoisomer (3D mirror image) of ALA (R-ALA). This means that in order to benefit maximally from these effects, R-ALA should be taken roughly 1 hour prior in a dose in the neighborhood of 30 mg/kg (2,700 mg for a 200 lb person)1,6; that is not to say that we will not reap some rewards from lesser doses. As is common of many therapeutic clinical trials, the doses are quite a bit higher (or far too low) than is required to see the benefits of the supplement.
If you want improved cellular energy production as well as enhanced utilization of insulin and GLUT4 to drive nutrients into the cells, then you should be supplementing with ALA!
Heck, you should be supplementing with ALA regardless of its nutrient partitioning effects. ALA will greatly benefit your health by reducing chronic inflammation and acute high blood pressure, acting as an antioxidant, as well as assisting your body in the removal of toxic heavy metals.
Throughout this review, I’ve given several doses of ALA supplementation ranging from 200 mg to 8,100 mg for 200 pound persons. I believe that a dose of up to 600 mg roughly an hour prior to eating or exercise will allow the best immediate benefits while a dose of around 1,000 mg per day will give you many of the longer term health benefits associated with ALA. Although ALA is both water and fat soluble, ingestion without food AND as the sodium-salt (Sodium lipoate) demonstrated a higher peak blood concentration as well as a longer half-life in the body40.
Of all the non-essential supplements out there, alpha-lipoic acid boats quite a stock of benefits for optimal health and performance. Now you know that this wonder supplement is doing much more than making your intraworkout nutrition and refeeds more effective!
Until next time!
Alden Ryno
Alden is a personal trainer, accredited nutritionist, and passionate lifestyle coach for the everyday, overworked person. At 20, he graduated from North Georgia College & State University in 2012 with a Bachelor’s in Chemistry. After which, he attended the Georgia Institute of Technology as a graduate student, but left upon completion of his PhD coursework in order to pursue his passion of helping others attain healthier lives. His company Red Ryno Athletics and website, xfitgen.com, are aimed at helping beginners gain ground on their fitness and life goals.
He’d love to hear from you at [email protected]
Visit his website: xfitgen.com
Twitter: @alderslodge
Instagram: @alden_ryno
1. R. S. Streeper , E. J. Henriksen , S. Jacob , J. Y. Hokama , D. L. Fogt HJT. Differential effects of lipoic acid stereoisomers on glucose metabolism in insulin-resistant skeletal muscle. Am J Physiol. 273(1):185-191. http://www.ncbi.nlm.nih.gov/pubmed/9252495.
2. Patrick L. Mercury toxicity and antioxidants: Part 1: role of glutathione and alpha-lipoic acid in the treatment of mercury toxicity. Altern Med Rev. 2002;7(6):456-471.
3. Shay K, Moreau R, Smith E. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys …. 2009;1790(10):1149-1160. doi:10.1016/j.bbagen.2009.07.026.Alpha-lipoic.
4. Gomes MB, Negrato CA. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol Metab Syndr. 2014;6(1):80. doi:10.1186/1758-5996-6-80.
5. V. A. Hughes , M. A. Fiatarone , R. A. Fielding , B. B. Kahn , C. M. Ferrara , P. Shepherd , E. C. Fisher , R. R. Wolfe , D. Elahi WJE. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol – Endocrinol Metab. 264(6):855-862. http://www.ncbi.nlm.nih.gov/pubmed/8333511.
6. Vitoon Saengsirisuwan , Felipe R. Perez , Julie A. Sloniger , Thomas Maier EJH. Interactions of exercise training and alpha-lipoic acid on insulin signaling in skeletal muscle of obese Zucker rats. Am J Physiol – Endocrinol Metab. 287(3):529-536. http://www.ncbi.nlm.nih.gov/pubmed/15068957.
7. J. Teichert, J. Kern, H.J. Trischler, H. Ulrich RP. Investigations on the pharmacokinetics of alpha-lipoic acid in healthy volunteers. Int J Clin Pharmacol Ther. 1998;36(12):625-628. http://www.ncbi.nlm.nih.gov/pubmed/9876998.
8. Woo Je Lee, Kee-Ho Song, Eun Hee Koh, Jong Chul Won, Hyoun Sik Kim, Hye-Sun Park, Min-Seon Kim, Seung-Whan Kim, Ki-Up Lee J-YP. Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem Biophys Res Commun. 2005;3(8):885-891. doi:10.1016/j.bbrc.2005.05.035.
9. YJ Suzuki, M Tsuchiya LP. Thioctic acid and dihydrolipoic acid are novel antioxidants which interact with reactive oxygen species. Free Radic Res Commun. 1991;15(5):255-263. http://www.ncbi.nlm.nih.gov/pubmed/1666623.
10. Robert L. Searls DRS. alpha-Ketoglutaric Dehydrogenase. 1Journal Biol Chem. 960;236(8):2485-2491. http://www.ncbi.nlm.nih.gov/pubmed/14444366.
11. Gerreke Ph. Biewenga, Guido R.M.M. Haenen AB. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol Vasc Syst. 29(3):315-331. doi:10.1016/S0306-3623(96)00474-0.
12. Liang-Jun Yan, Maret G. Traber, Hirotsugu Kobuchia, Seiichi Matsugo, Hans J. Tritschler LP. Efficacy of hypochlorous acid scavengers in the prevention of protein carbonyl formation. Arch Biochem Biophys. 1996;327(2):330-334. http://www.ncbi.nlm.nih.gov/pubmed/8619623.
13. A. Elisabeth Hak, Huibert A. P. Pols, Coen D. A. Stehouwer, John Meijer, Amanda J. Kiliaan, Albert Hofman, Monique M. B. Breteler and JCMW. Markers of Inflammation and Cellular Adhesion Molecules in Relation to Insulin Resistance in Nondiabetic Elderly: The Rotterdam Study. J Clin Endocrinol Metab. 2001;89(6):4398-4405. http://press.endocrine.org/doi/abs/10.1210/jcem.86.9.7873?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dpubmed&.
14. Peimian Ou, Hans J. Tritschler SPW. Thioctic (lipoic) acid: a therapeutic metal-chelating antioxidant? Biochem Pharmacol. 1995;50(1):123-126. http://www.ncbi.nlm.nih.gov/pubmed/7605337.
15. Hagen JHSRMS-HDHTM. Dietary supplementation with (R)-alpha-lipoic acid reverses the age-related accumulation of iron and depletion of antioxidants in the rat cerebral cortex. Redox Rep. 2005;10(1):52-60. doi:10.1179/135100005X21624.
16. Craig J. McMackin, Michael E. Widlansky, Naomi M. Hamburg, Alex L. Huang S, Weller, Monika Holbrook, Noyan Gokce TMH, , John F. Keaney Jr. and JA, Vita. Effect of Combined Treatment with Alpha Lipoic Acid and AcetylL-Carnitine on Vascular Function and Blood Pressure in Coronary Artery Disease Patients. J Clin Hypertens. 2009;27(5):417-428. doi:10.1055/s-0029-1237430.Imprinting.
17. S Vasdec, V Gill, L Longerich, S Parai VG. Salt-induced hypertension in WKY rats: prevention by alpha-lipoic acid supplementation. Mol Cell Biochem. 2003;254(1-2):319-326. http://www.ncbi.nlm.nih.gov/pubmed/14674712.
18. S Vasdev, CA Ford, S Parai, L Longerich VG. Dietary lipoic acid supplementation prevents fructose-induced hypertension in rats. Nutr Metab Cardiovasc Dis. 2000;10(6):339-346. http://www.ncbi.nlm.nih.gov/pubmed/11302009.
19. TORY M. HAGEN, RÉGIS MOREAU JHS andFRANCESCO V. Mitochondrial decay in the aging rat heart: evidence for improvement by dietary supplementation with acetyl-L-carnitine and/or lipoic acid. Ann N Y Acad Sci. 2002;959:491-507. doi:10.1111/j.1749-6632.2002.tb02119.x.
20. Thomas Heitzer, Barbara Finckh, Sylvia Albers, Karoline Krohn, Alfried Kohlschütter TM. Beneficial effects of alpha-lipoic acid and ascorbic acid on endothelium-dependent, nitric oxide-mediated vasodilation in diabetic patients: relation to parameters of oxidative stress. Free Radic Biol Med. 2001;31(1):53-61. http://www.ncbi.nlm.nih.gov/pubmed/.
21. D Ziegler, M Hanefeld, K J Ruhnau, H Hasche, M Lobisch, K Schütte, G Kerum and RM. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a 7-month multicenter randomized controlled trial (ALADIN III Study). ALADIN III Study Group. Alpha-Lipoic Acid in Diabetic Neuropathy. Diabetes Care. 1999;22(8):1296-1301. http://www.ncbi.nlm.nih.gov/pubmed/10480774.
22. H Schupke, R Hempel, G Peter, R Hermann, K Wessel, J Engel TK. New metabolic pathways of alpha-lipoic acid. Drug Metab Dispos Biol Fat Chem. 29(6):855-862. http://www.ncbi.nlm.nih.gov/pubmed/11353754.
23. L Packer, EH Witt HT. alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med. 19(2):227-250. http://www.ncbi.nlm.nih.gov/pubmed/7649494.
24. A Bast GH. Interplay between lipoic acid and glutathione in the protection against microsomal lipid peroxidation. Biochem Biophys acta. 1988;963(3):558-561. http://www.ncbi.nlm.nih.gov/pubmed/3143421.
25. E Busse, G Zimmer, B Schopohl BK. Influence of alpha-lipoic acid on intracellular glutathione in vitro and in vivo. Arzneimittelforschung. 1992;42(6):829-831. http://www.ncbi.nlm.nih.gov/pubmed/1418040.
26. Han D, Handelman G, Marcocci L, Sen CK, Roy S, Kobuchi H, Tritschler HJ, Flohé L PL. Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization. Biofactors. 1997;6(3):321-338. http://www.ncbi.nlm.nih.gov/pubmed/9288403.
27. B Anuradha PV. Protective role of DL-alpha-lipoic acid against mercury-induced neural lipid peroxidation. Pharmacol Res. 1999;39(1):67-80. http://www.ncbi.nlm.nih.gov/pubmed/10051379.
28. Grunert R. The effect of DL-alpha-lipoic acid on heavy-metal intoxication in mice and dogs. Arch Biochem Biophys. 1960;86:190-194. http://www.ncbi.nlm.nih.gov/pubmed/13829866.
29. Shay KP, Moreau RF, Smith EJ, Hagen TM. Is α-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity. IUBMB Life. 2008;60(6):362-367. doi:10.1002/iub.40.
30. Federici M, Menghini R, Mauriello A, et al. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106(4):466-472. doi:10.1161/01.CIR.0000023043.02648.51.
31. Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):1-10. doi:10.1101/cshperspect.a001651.
32. Tak PP, Firestein GS, Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7-11. doi:10.1172/JCI11830.
33. Kunt T, Forst T, Wilhelm a, et al. Alpha-lipoic acid reduces expression of vascular cell adhesion molecule-1 and endothelial adhesion of human monocytes after stimulation with advanced glycation end products. Clin Sci (Lond). 1999;96(1):75-82.
34. Kim H, Kim H, Park K, et al. α-Lipoic acid inhibits matrix metalloproteinase-9 expression by inhibiting NF-κB transcriptional activity. Exp Mol Med. 2007;39(1):106-113.
35. Hubert Schupke, Roland Hempel, Gernot Peter, Robert Hermann, Klaus Wessel JE and TK. New metabolic pathways of alpha-lipoic acid. Drug Metab Dispos Biol Fat Chem. 2001;29(6):855-862. http://www.ncbi.nlm.nih.gov/pubmed/11353754.
36. Kyung-Joo Cho, Hadi Moini, Hee-Kyung Shon, An-Sik Chung LP. α-Lipoic acid decreases thiol reactivity of the insulin receptor and protein tyrosine phosphatase 1B in 3T3-L1 adipocytes. Biochem Pharmacol. 2003;5(1):849-858. doi:10.1016/S0006-2952(03)00395-2.
37. Ojuka EO. Role of calcium and AMP kinase in the regulation of mitochondrial biogenesis and GLUT4 levels in muscle. Proc Nutr Soc. 2004;63(2):275-278. doi:10.1079/PNS2004339.
38. Raynald Bergeron , Raymond R. Russell III, Lawrence H. Young , Jian-Ming Ren , Melissa Marcucci , Agnes Lee GIS. Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am J Physiol. 1999;276(5):938-944. http://www.ncbi.nlm.nih.gov/pubmed/10329989.
39. Winder WW. Energy-sensing and signaling by AMP-activated protein kinase in skeletal muscle. J Appl Physiol. 2001;91(3):1017-1028. http://www.ncbi.nlm.nih.gov/pubmed/11509493.
40. D.A. Carlson, A.R. Smith, S.J. Fischer, K.L. Young LP. The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)-lipoate to healthy human subjects. Altern Med Rev. 2007;12(4):434-451. http://www.ncbi.nlm.nih.gov/pubmed/18069903.
41. Min-Seon Kim, Joong-Yeol Park, Cherl Namkoong, Pil-Geum Jang, Je-Won Ryu, Hai-Sun Song, Ji-Young Yun, Il-Seong Namgoong, Joohun Ha, In-Sun Park, In-Kyu Lee, Benoit Viollet, Jang Hyun Youn H-KL& K-UL. Anti-obesity effects of α-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727-733. doi:10.1038/nm1061.